Overview
Artwork Content to Carton Services - What you communicate is what the end-user reacts to.
In Life Sciences, communication refers to the safety information conveyed and the visual appeal of products inclusive of:
- The label content, which should be compliant with the artwork guidelines for packaging set by different health authorities
- The content creation for artwork, which should be appealing and aligned with the global market sensibilities
- The artwork prints, which should be on par with the global standards
- The packed carton, should stand out and should possess all the safety standards
- The expertise of resources for each of the areas related to Artwork Lifecycle Management
With the rise in demand for globalization and market expansions, pharmaceutical manufacturers, of late, are forced to carry out all these activities at various locations, if not being assisted by globally distributed partners/vendors. Hence, the challenge persists in gaining comprehensive visibility into end-to-end processes which otherwise may lead to operational delays and affect the product’s time to market.
Freyr, with a strong foothold in the life sciences Regulatory landscape and its expertise in Artwork content to carton services, is capable to handle end-to-end operational activities right from content until the carton or from artwork prints to manufacturing sites for packaging. The concept of Content to Carton is about the seamless integration of all the Regulatory departments and artwork and pack management systems, starting from dossier creation to SmPC document to content creation for artwork for submission and commercialization until it reaches the artwork print vendor, with the objective of right content going out to Regulatory bodies and to consumers buying the product.
Freyr’s content-to-carton team is specialized in the creation of label content, tracking technical change specifications, content creation for artworks, Prepare Packaging Artwork for Printed Cartons, managing artwork launch requests, integrating Regulatory text changes, partnering with printers for accurate carton printing, and handing over Printed Packaging Material (PPM) to the packaging team while adhering to the Artwork Guidelines for Packaging and Artwork Guidelines for Folding Carton along with the best safety standards.
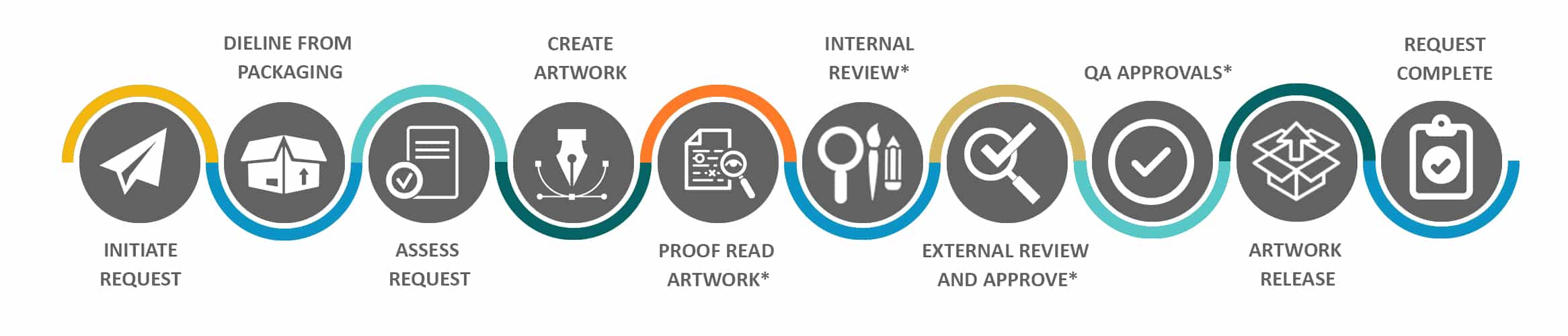
Process Steps & Functions Involved in Commercial Request
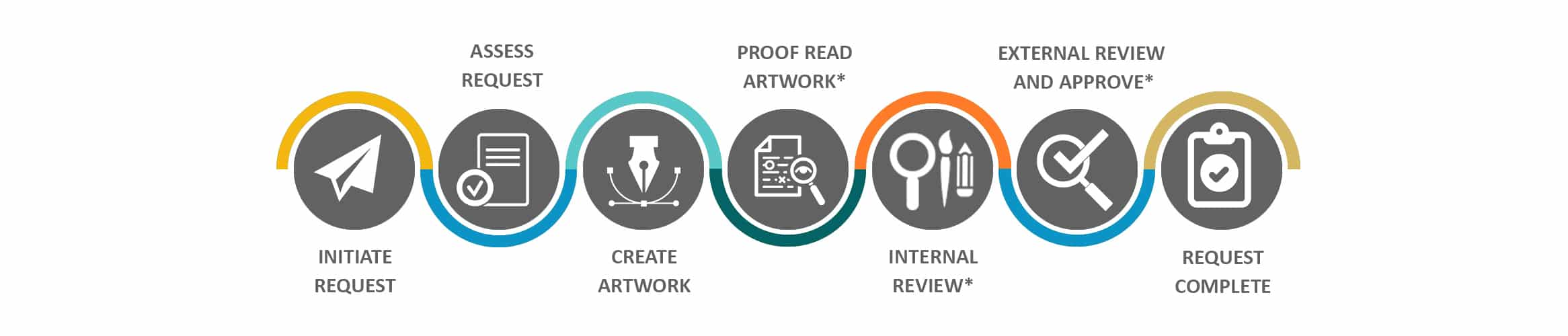
Process Steps & Functions Involved in Submission Request
Artwork Content to Carton Services - Freyr Expertise
- Content creation for Labeling and Artwork.
- Pharmaceutical artwork management.
- Content submission to Health Authorities (HAs) as part of the dossier.
- Submission of sample artwork as part of the dossier.
- Submission of artwork change management based on HA reviews.
- Creating commercial artworks for the intended local markets:
- Integrating the change request in a specialized in-house tool.
- Coordinating with stakeholders for all inputs.
- Artwork studio services.
- Proofreading and e-proofing services.
- Regulatory affairs review and approval.
- Marketing review and approval.
- Packaging review and approval.
- Quality review and approval.
- Valued partnerships for artwork printing.
- Quality check of incoming packaging artwork.
- Tracking technical change specifications.
- Integrating Regulatory text changes.


Artwork Content to Carton Services - Freyr Advantages
- End-to-end visibility.
- Global pool of Regulatory artwork experts.
- Artwork Lifecycle Management.
- One-stop-shop for all labeling content to printed packaging material.
- Assured accuracy to mitigate product recalls.
- Quick turnaround time (TAT).
- Product & brand credibility mitigating branding inconsistencies.
- 24/7 artwork pack management operations.
- Multi-region/market support.
- Multi-lingual artwork creation.


